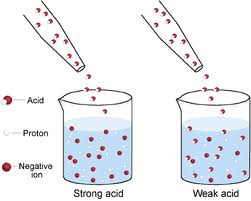
Acid Dissociation Constant An acid is ionizes partially in aqueous solutions is a weak acid. The strengths of weak acid can be compared using their dissociation constants. A weak acid ionizes partially as follows.HA (aq) H+ (aq) + A-(aq) Applying the law of mass action to the above equilibrium,The weak acid dissociation constant is the ratio of the product of molar concentration of the product of ionization to the molar concentration of the unionized acid at equilibrium. The larger the value of Ka, the stronger is the acid.
|
|







